A metal comprises of uncharged blocks known as atoms. When a metal corrodes in a corrosive environment, atoms with positive charge flow from the metal surface and dissolve in the liquid.
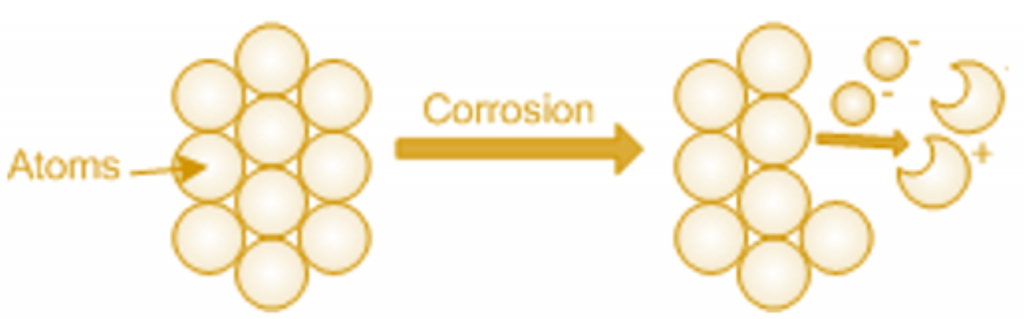
Negatively charged electrons remain in the metal. If free oxygen (O2) or positively charged hydrogen atoms (H+) are contained in the liquid, the electrons are consumed in the metal, and corrosion proceeds. Reactions of metal (Me) with oxygen can be shown as:
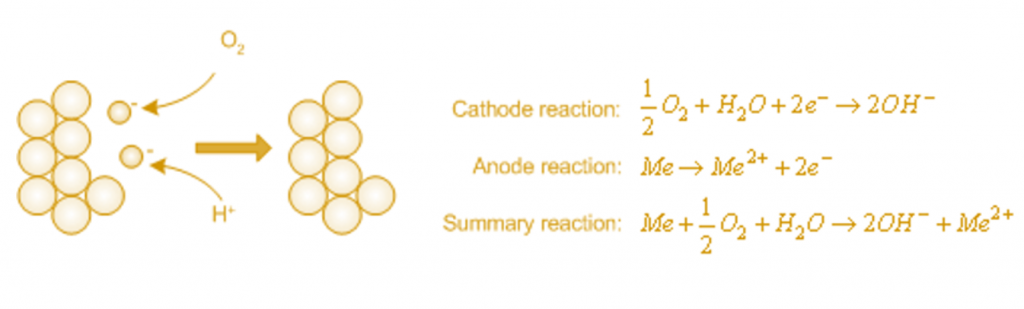
If the electrons are not consumed, the negative charges become so high that the atoms cannot leave the surface of the metal because of the electrostatic forces of attraction.
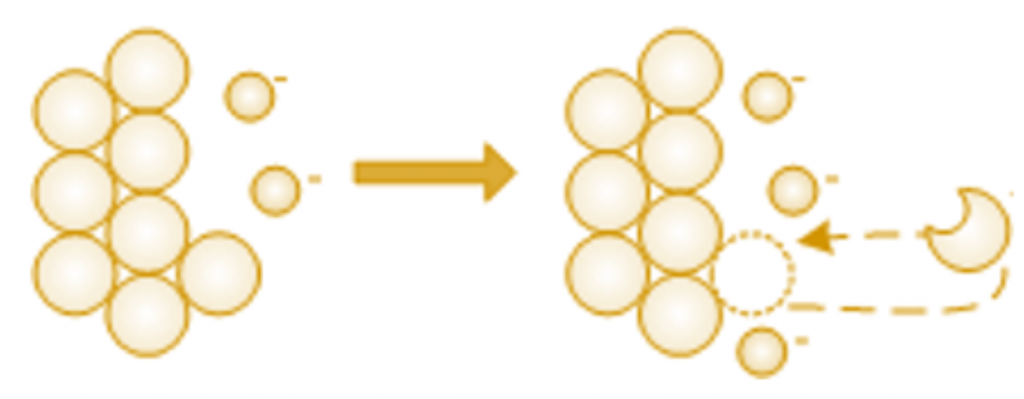
Corrosion can be prevented by eliminating oxygen or the positively charged hydrogen ions. But this cannot be carried out in practice. One of the better methods is to create an excess of electrons in the metal so that positively charged metal atoms can stay on the surface of the metal because of the electrostatic forces of attraction.
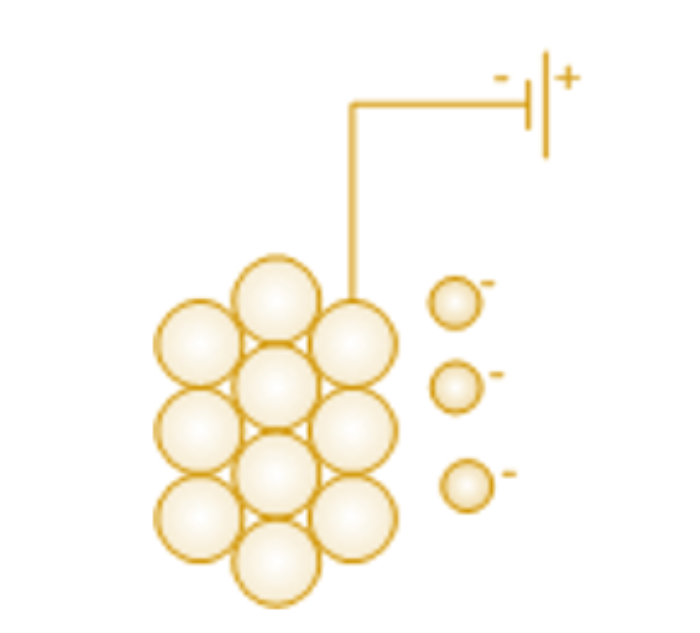
Cathodic protection is a method to prevent corrosion on the metal surface.
Methods of Cathodic Protection
There are two ways of cathodic protection. One method uses sacrificial anodes while the other method uses impressed current from an external source.
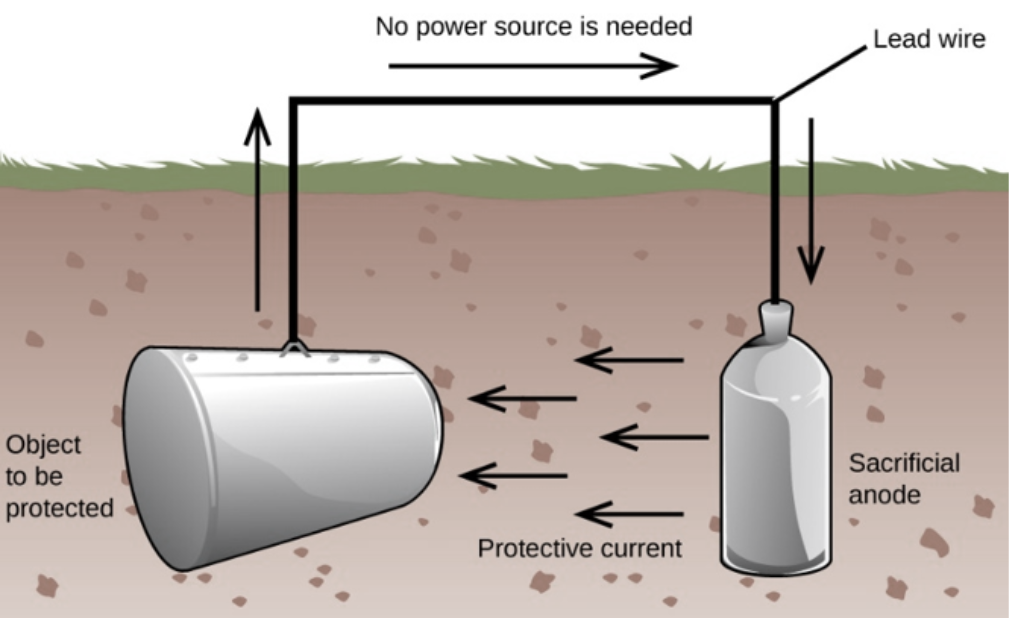
1. Sacrificial/Galvanic Anodes
To understand the working of the sacrificial anodes for cathodic protection it is required to have in mind the galvanic series of metals. Metals become more electronegative with the increase in the tendency of losing ions in the solution. Zinc, aluminum, and magnesium are more electronegative than steel they are able to supply electrons to the steel when in electrical contact with water and will affect the cathodic protection of the steel surface.
If steel is coupled to copper in saltwater, steel would supply the electrons to the copper which would become protected and the corrosion of the steel would be enhanced in this environment. Electrons are supplied to the steel pipe via electrical connections, and a corresponding amount of anode material goes into the solution as metal ions.
Read also: Corrosion and its Mitigation Techniques
Unlikely the impressed-current anodes, the sacrificial anodes must be located close to the structure being protected. Although any piece of the anode could provide cathodic protection over a short period of time, cathodic protection schemes are typically required to operate for several years. Over a period of time, anodes lose their activity and develop a non-conducting film on their surfaces that prevents the current supply. This is normally avoided by careful control of the concentrations of additive trace impurities in the anode materials by alloying.
2. Impressed Current
The arrangement for protecting a buried pipeline is illustrated in Fig 3. The pipe receives the current from a direct current power source via an auxiliary electrode buried in the ground. The pipe becomes the cathode and the auxiliary electrode becomes the anode. If the anode is an electrochemically inert material, the surrounding environment will be oxidized. The following materials have been used as anodes: magnetite, graphite, high silicon iron, lead/lead oxide, and platinized materials. Platinum has a very high resistance to corrosion and it would be an ideal anode material but its high cost is its major disadvantage.
2.1. Parts of Impressed
The main components of an impressed current cathodic protection system are anodes, backfill of the anode, a power supply, a structure to be protective, electrical wiring, and connections. The anodes used in impressed current cathodic systems are different from those used in sacrificial systems. Impressed current anodes are manufactured from materials that are consumed at relatively low rates. Impressed current cathodic protection systems generally operate at higher current and driving voltage levels than sacrificial anode cathodic protection systems. The anode for cathodic protection is not in direct contact with the soil in which it is buried because the minerals and other chemicals of the soil can affect the anode and can decrease its effectiveness.
In addition, we require the anode to get uniformly consumed with maximum efficiency. That’s why special backfills are used depending on the environment and the anode material. The prime purpose of using the backfill is to increase electrical conductivity. This lowers the anode-to-earth resistance and greater current outputs in cases where the surrounding soil is of high resistivity.

1 Comment
Corrosion and its Mitigation Techniques - Chemiopedia · December 6, 2022 at 10:06 pm
[…] Read more: Cathodic Protection and its two types […]
Comments are closed.