Combustion is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. ¨The release of heat can produce light in the form of either glow or a flame.
Complete combustion is almost impossible to achieve and products of incomplete combustion may be present, such as carbon monoxide, and carbon, in the flue gases. Additionally, any combustion at high temperatures in atmospheric air, which is 79% N2, will also create small amounts of several nitrogen oxides, commonly referred to as NOx.
You may like to read: Fire Types and Fire Extinguishers
The following figure shows the fire triangle. This triangle illustrates the three elements a fire needs to ignite: heat, fuel, and an oxidizing agent (usually air or oxygen). A fire occurs naturally when these elements are present in the right ratios. A fire can be extinguished by removing any one of these elements in the fire triangle.
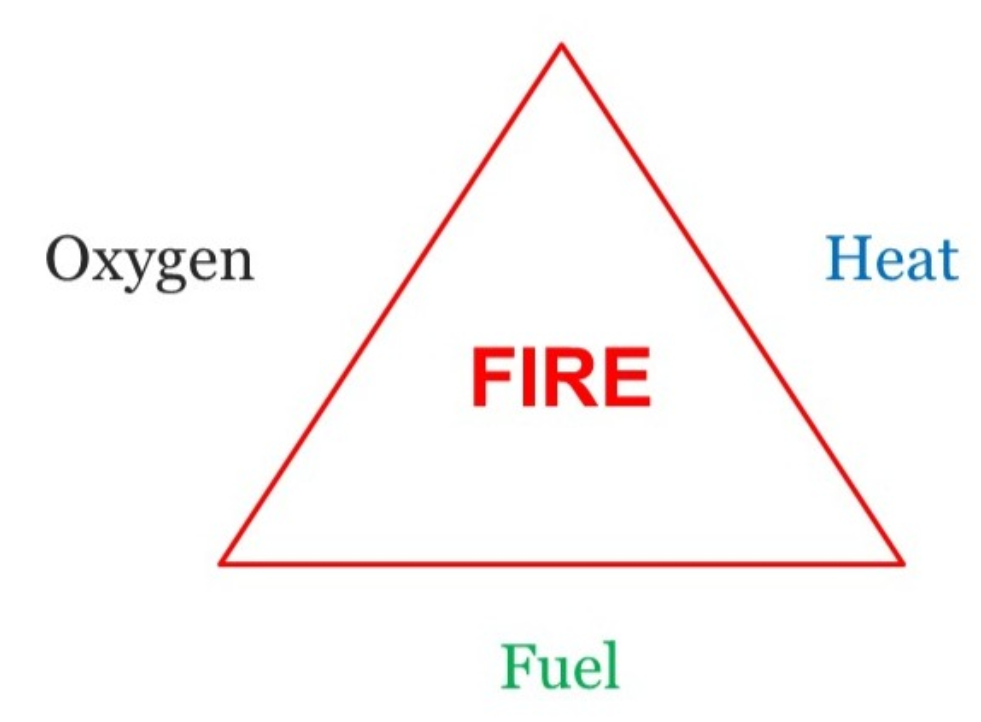
For example, covering flames with the fire blanket blocks oxygen and can extinguish a fire.
Types of Combustion
1. Complete Combustion
In complete combustion, the reactant burns in oxygen, producing a limited number of products. When a hydrocarbon burns in oxygen, the reaction will primarily yield carbon dioxide and water. When elements are burned, the products are primarily the most common oxides. Carbon will yield carbon dioxide, sulfur will yield sulfur dioxide, and iron will yield iron(III) oxide.
2. Incomplete Combustion
Incomplete combustion will occur when there is not enough oxygen to allow the fuel to react completely to produce carbon dioxide and water. It also happens when the combustion is quenched by a heat sink, such as a solid surface or a flame trap. The product of incomplete hydrocarbon combustion includes carbon monoxide and carbon.
3. Rapid Combustion
Rapid combustion/explosion is a form of combustion, otherwise known as a fire, in which large amounts of heat and light energy are released, which often results in a flame. This is used in a form of machinery such as internal combustion engines and in weapons.
Three T’s of Combustion
The objective of good combustion is to release all of the heat in the fuel. This is accomplished by controlling the “three T’s” of combustion which are:
- Temperature high enough to ignite and maintain ignition of the fuel
- Turbulence or intimate mixing of the fuel and oxygen
- Time, sufficient for complete combustion
Combustion Stoichiometry
Air is the common oxidizing agent in a combustion reaction. The reaction equation of hydrocarbon in the presence of air can be written as:
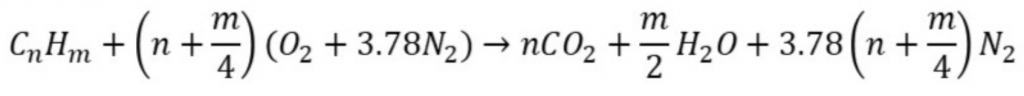
while n and m are the carbon and hydrogen moles in the respective hydrocarbon. Chemiopedia owes an android mobile application to calculate the:
- Hydrocarbon mixture gross and net heating values
- Oxidant flow calculation for complete combustion.
- Flue gas composition for the given fuel and oxidant flow.
- Excess air/flame temperature graphical representation.
This application can help users to quickly estimate the combustion stoichiometry for their hydrocarbon mixture.
Download the application: Combustion Stoichiometry