Conduction is the way of energy transfers between two bodies at different temperatures. It is due to the diffusion process by which thermal energy spreads from hotter regions to cooler regions of a solid or stationary fluid. Gebhart et al (1993). observed that the overall heat conduction may be the sum of the number of individual effects such as molecular and electron diffusion.
Read also: Classification of Heat Exchangers
Example
- Heat transferred between the electric stove and the bottom of a pot.
- The heat from tea makes the cup hot.
- A heat exchanger uses a hot fluid to conduct heat to a cooler fluid without the two touchings.
- Sand can conduct heat. Walking on the beach on a hot summer day will warm your feet.
Fourier Law of Heat Conduction
The negative gradient of temperature and the time rate of heat transfer is proportional to the area at right angles of that gradient through which the heat flows and inversely proportional to the thickness of the conductor, is known as Fourier’s law of heat conduction.
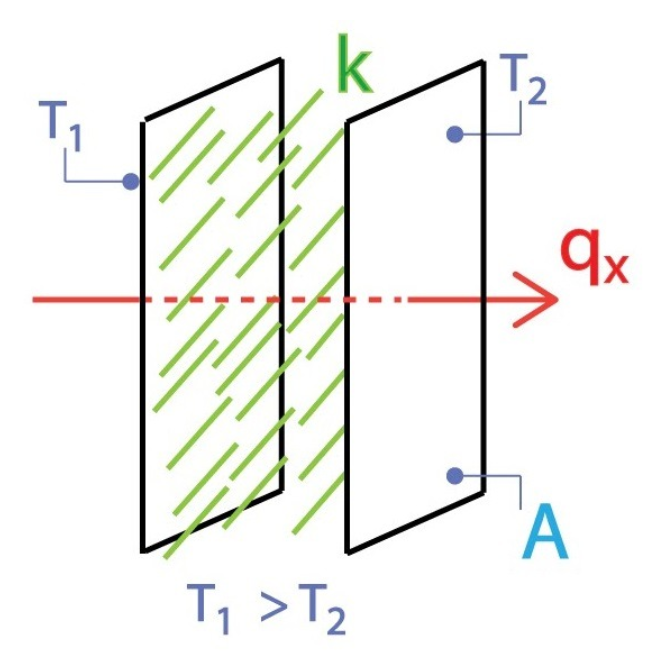
Consider a plate at temperature T1 and a second plate at temperature T2. Both plates have a cross-sectional area A. Temperature T1 is greater than temperature T2. A conducting material is placed between two plates with thermal conductivity k. If we assume the unidirectional heat flow the qx represents the conductive heat flux between plates through the conductive material.
According to the definition:
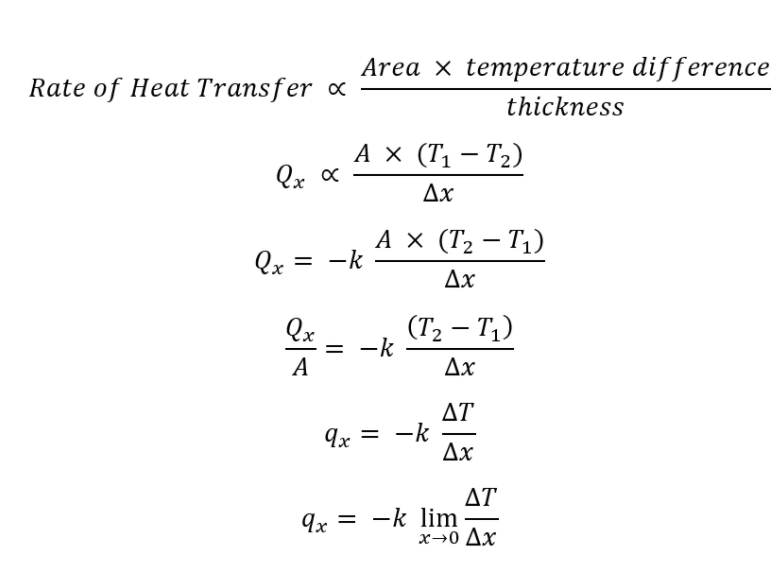
The final unidirectional mathematical form of Fourier law is represented as:
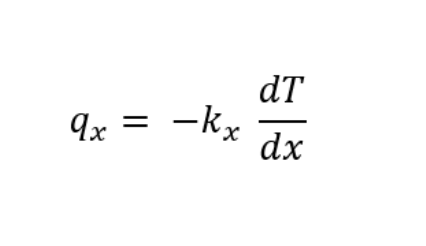
For three-dimensional heat flux:
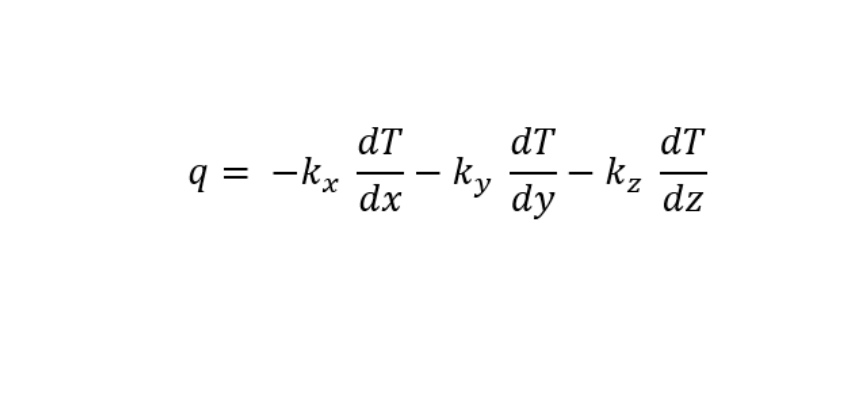
While kx, ky, and kz is the thermal conductivity of the conductor in x, y, and z-direction respectively. If the material is an isotropic material then:
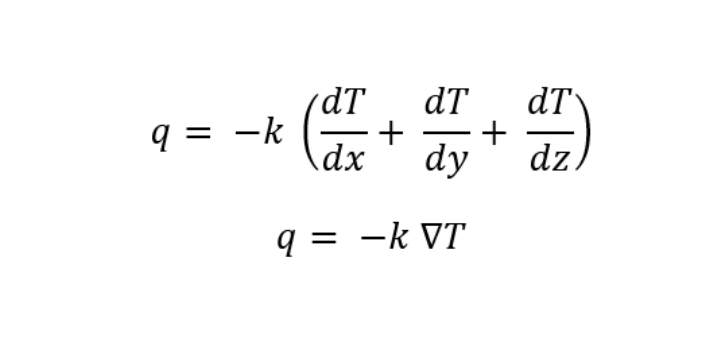
1 Comment
Fourier’s Law of Heat Conduction - Chemiopedia · December 1, 2022 at 7:24 pm
[…] See: Conduction […]
Comments are closed.