Hydrates are generally classified by the arrangement of the water molecules within the crystal structure. There are two types of hydrates commonly encountered in the petroleum industry.
1. Type I Hydrates
Type-I is the simplest structure hydrates. It is made of two types of cages:
- Dodecahedron
- Tetrakaidecahedron
The first one is a 12-sided polyhedron having each face as a regular pentagon while the second type has a 14-sided polyhedron having 12 pentagonal and two hexagonal faces. The first cage is smaller than the second cage.
This type of hydrate consists of forty-six (46) molecules of water. If Y is represented as hydrate former then the theoretical formula for the hydrate can be expressed as:
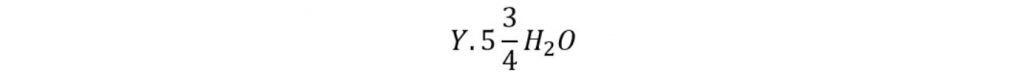
Methane, ethane, hydrogen sulfide, and carbon dioxide are the common type I hydrate former.
Read also: Nitrogen Rejection Process
2. Type II Hydrates
Type II hydrates have significantly more complex than type I hydrates. Type II hydrates structure also have two types of cages:
- Dodecahedron
- Hexakaidecahedron
The former cage is the same as in type I while the second cage has the 16-sided polyhedron having 12 pentagonal and 4 hexagonal faces. The hexakaidecahedron cages are bigger than the dodecahedral cages.
This type of hydrate consists of 136 water molecules. If Y is represented as hydrate former then the theoretical formula for the hydrate can be expressed as:
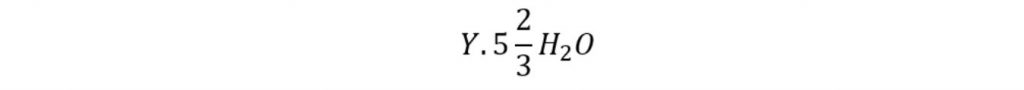
If only large cages are occupied by the guest molecule then the theoretical formula is expressed as:
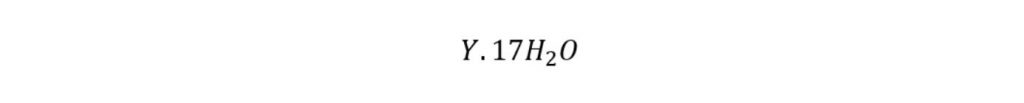
Propane, isobutane, and nitrogen are the common type II hydrate former.