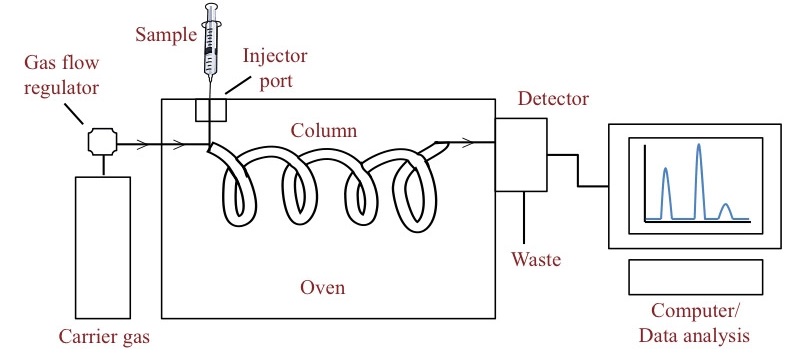
Chromatography is an important analysis in the industry. It is used as an online analyzer as well as a laboratory analyzer. The installation of a chromatography system requires some consideration.
- Analytical Components of Interest
- The Analytical Method
- Carrier Gas
- Sample collection and preparation
- Sample injection in the chromatograph
- Column Selection
- Component detectors
- Detector signal integration and data handling
- Ventilation and area classification
- Calibration and Verification
- Training of personnel and Follow-up
- Maintenance and Trouble Shooting
The gas Processors Association (GPA) provides technical standards for chromatography systems.
- GPA 2165: Method for Analysis of Natural Gas Liquid Mixtures by Gas Chromatography
- GPA 2177: Method for the Analysis of Demethanized Hydrocarbon Liquid Mixtures Containing Nitrogen and Carbon Dioxide by Gas Chromatography
- GPA 2186: Tentative Method for the Extended Analysis of Demethanized Hydrocarbon Liquid Mixtures Containing Nitrogen and Carbon Dioxide by Temperature Programmed Gas Chromatography
- GPA 2261: Method for Analysis of Natural Gas and Similar Gaseous Mixtures by Gas Chromatography
- GPA 2286: Tentative Method for the Extended Analysis
- of Natural Gas and Similar Gaseous Mixtures by Temperature Programmed Gas Chromatography
- GPA 2199: The Determination of Specific Sulfur Compounds by Capillary Gas Chromatography and Sulfur Chemiluminescence Detection
- GPA 2198: Selection, Preparation, Validation, Care, and Storage of Natural Gas and Natural Gas Liquids Reference Standard Blends
The Product Stream and Components of Interest
The selection of a chromatography system is initially based on product stream composition and components of interest. However, this step is not considered in true spirit as it should be for the selection. Typical samples must be analyzed for every significant component. All possible plant configurations and operating conditions should be considered as they may cause variation in the stream composition.
Additionally, the seasons of the location may also consider while selecting the typical composition. The components of interest do not necessarily include all the components in the stream. Sometimes minor components are included in a single peak say C6+ components are included in a single peak in the natural gas processing plant. The analysis time is based on the required accuracy of the analysis. If C6+ components are required, then the chromatograph will require an extended analysis time for the unit sample.
Read also: Natural gas sampling
Carrier Gas
The carrier gas is an important component of the chromatograph. It carries the liquid/gas sample through the chromatography system from the sample injection system to the detector via a separating column. Helium and hydrogen are typically used as carrier gas due to their high thermal conductivity.
Sample Preparation and Introduction
Sample preparation is an essential step to produce true representative results. The sample should either be in fully liquid or fully vapor form to get good results. The two-phase sample may introduce variation in the results due to different fractions of components in both phases.
In natural gas analysis, a vaporizer regulator is installed to increase the temperature and reduce the pressure of the gas. This ensures the complete vaporization of the sample before injection into the column. The transfer line should also be heat traced to avoid condensation within the sample. The gas sample is generally heated up to 140°F before analysis.
The liquid sampling loop transfers the sample from the sample point to the chromatograph for analysis and back to the lower pressure location in the product piping network. Filtration and moisture removal from the sample is an important considerations for the analysis. The sample must be dehydrated without changing its composition. Sample tubing should be small enough to have a large velocity of the sample. Larger diameters tend to produce historical results.
In laboratory systems, it is important that the sample in the sample cylinder is either completely in the liquid phase or completely in the vapor phase. In a two-phase state, the vapor portion will have a higher concentration of the more volatile (light ends) components than the sample and the liquid phase will have a higher concentration of the less volatile (heavy ends) components.
A sample withdrawn from the top or from the bottom of the sample cylinder will not truly represent the composition of the entire cylinder. Sample withdrawal from either phase will irrevocably change the composition of the sample left in the cylinder. Natural convection of a heated, vaporized sample will keep the sample mixed and homogenous. Samples that are completely liquid must be mechanically mixed to ensure a homogenous sample for analysis.
The introduction of a natural gas sample into the chromatograph includes drawing a vacuum on the sample tubing to assist in vaporizing and removing residual components from previous samples. The sample loop is then purged (typically at (or below) atmospheric pressure) through the sample loop before injection. It is necessary to maintain pressure well above bubble point pressure on the liquid sample during sample withdrawal. This can be achieved by using an appropriate displacement me-
A block valve/purge control valve system is installed downstream of the liquid sample valve to maintain pressure on the liquid sample until the sample is injected. This system is also used to purge the sample through the sample valve. It may be necessary to install filters and moisture traps in the sample lines that lead to the gas or liquid sample valves.
Of course, the filters and traps must not alter the composition of the sample. Since most of the troubleshooting problems in a chromatographic system are associated with sample preparation and sample introduction, it is very important to design or select this portion of the system with care.
Column Selection
The separating column is the most important part of the chromatograph. It separates the different components of the mixture sample. Its selection and installation require expertise. General industrial practices are considered the first approach to column selection. The vendor of the chromatograph provides a wide catalog of the columns that can be selected on a requirement basis. The column can be of two types:
- Packed-used for large sample volume
- Capillary-used for efficient separation
Optimum column temperatures and carrier gas flow must be determined as the inappropriate value of these may reduce the sharpness of resolution. Increasing temperature has the same effect as increasing flow. A combination of columns can also be used to get the desired results. Special design valves direct the sample to the different columns at different analysis times.
Low boiling point components are separated first in the column and are usually separated from each other at lower column temperatures. Higher boiling point components separate rather slowly and increased temperatures help to speed up the analysis process.
Detectors
Detectors are the last part of the chromatography system. They detect the different components of gas based on their properties. There are two major types of detectors:
Thermal Conductivity Detector
It is considered a universal detector for the chromatograph. It detects any component that has a thermal conductivity different from the carrier gas. Helium is the most frequently used carrier gas due to its high thermal conductivity (after hydrogen).
The thermal conductivity detector is a heated wire filament or thermistor bead. Its electrical conductivity is partially determined by its temperature. As product components elute from the end of the column, they cause the detector temperature to increase as compared to the temperature of a filament with pure carrier gas flowing.
This changes the electrical conductivity and the changed electrical signal is electronically interpreted by the integrator or computer system to produce a chromatogram peak whose area is proportional to the composition of the component.
The thermal conductivity detector is not extremely sensitive. It may not be the detector of choice for trace components.
Flame Ionization Detector
The flame ionization detector is more sensitive than the thermal conductivity detector for hydrocarbons and many other materials. This detector is not sensitive to inert components found in natural gases and natural gas liquids. (say Carbon Dioxide and Nitrogen). The effluent from the column flows through a hydrogen-air flame. The flame jet is electrically charged.
As the hydrocarbons are broken up in the flame, ionized particles are formed. These particles then flow across an electrically charged collector that develops an electrical signal proportional to the concentration of the component. There are many specialized detectors that selectively detect only certain components such as sulfur or halogens.
2 Comments
Natural Gas Sampling Method and Points - Chemiopedia · September 19, 2022 at 10:50 pm
[…] Read also: Natural gas and liquid chromatography […]
Well Head and Xmas Tree of Production Wells - Chemiopedia · December 3, 2022 at 4:19 pm
[…] Read also: Natural Gas and Liquid Chromatography […]
Comments are closed.