The Peng-Robinson equation of state is used for calculating the thermodynamic properties of both pure fluids and fluid mixtures. Only the critical temperature and pressure and the acentric factor are needed to determine the properties of pure fluid. Binary interaction parameters are needed in addition to determining the properties of a non-ideal mixture. The Peng-Robinson equation of state is claimed to provide reasonably accurate estimates of liquid as well as vapor phase densities. With known binary interaction parameters, the Peng-Robinson equation can be used to do multicomponent liquid-vapor phase equilibrium calculations.
Read also: Van der Wall Equation
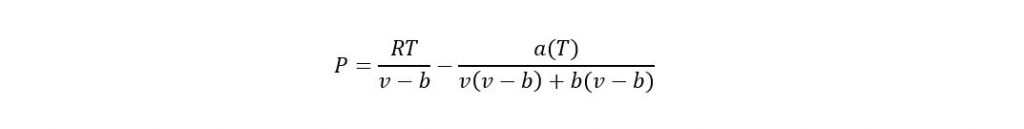
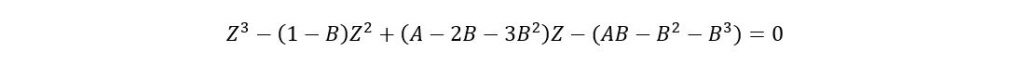
Where
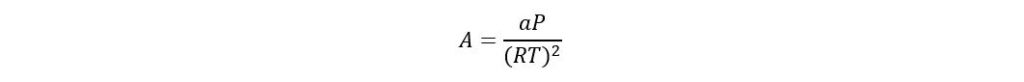
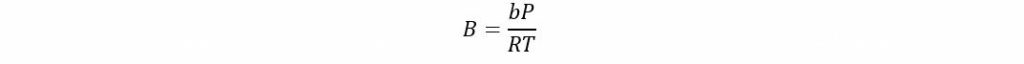
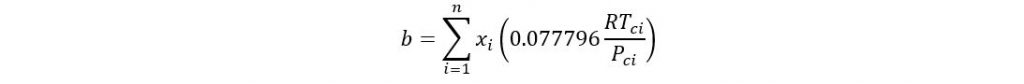
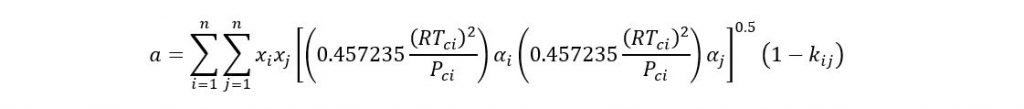
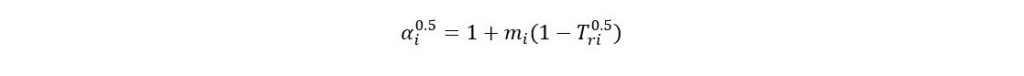
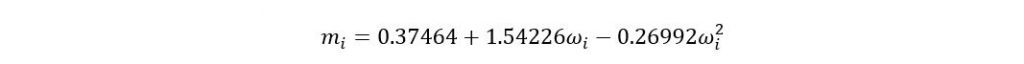
If acentric factor > 0.49 then

1 Comment
Van der Waals Equation - Chemiopedia · October 3, 2022 at 4:11 pm
[…] Read also: Peng Robinson Equation of State […]
Comments are closed.