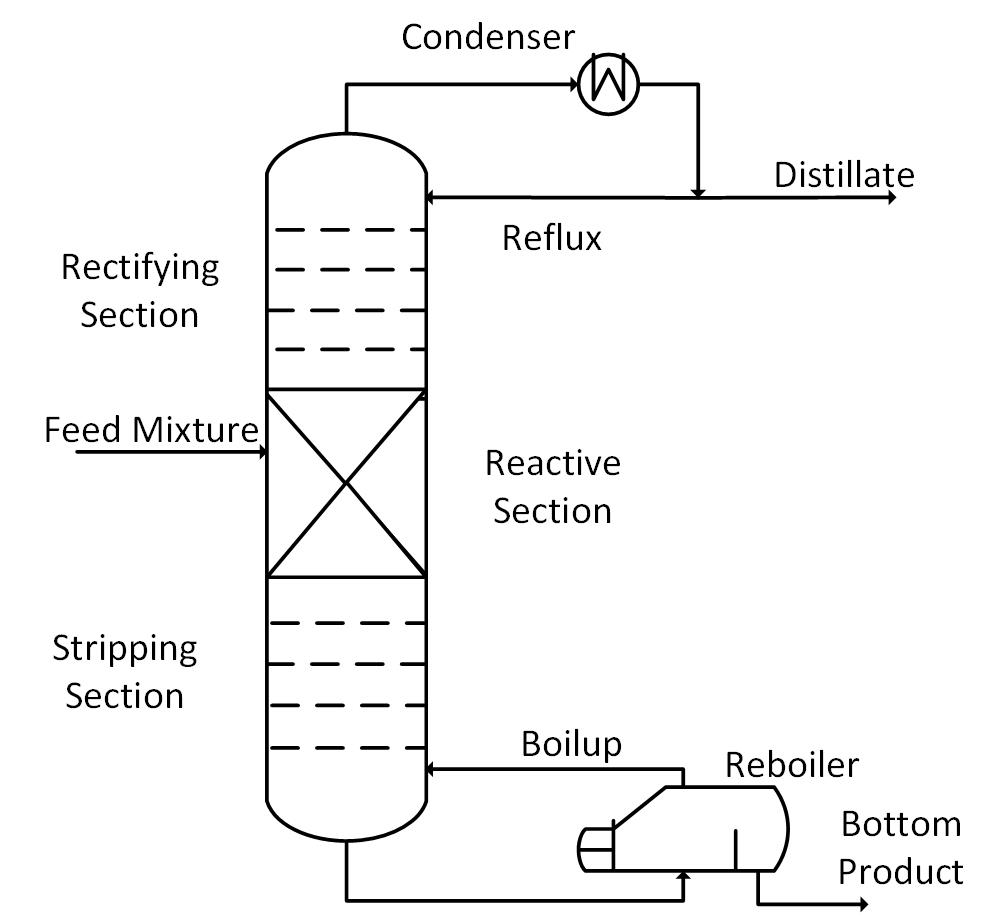
Reactive distillation is an integrated operation that combines the catalytic chemical reactions and the distillation in a single unit. This type of distillation is the ideal method for the reactions that are problematic to drive to completion without the continuous separation of one of the products. Such reactions are referred to as ‘equilibrium limited’. Simultaneous production and removal of products improve productivity and selectivity.
Reactive distillation is also a cost-effective process because the separation of the product from the reaction mixture does not need a separate distillation unit. This technique is useful for esterification and ester hydrolysis reactions because they are equilibrium-limited.
Example
The esterification of acetic acid with alcohols including methanol, n-butanol, ethanol, isobutanol, and amyl alcohol.
Read also: Packed column and its types
Constraints of Reactive Distillation
Reactive Distillation can be used in removing acetic acid from water.
- There are four important constraints for applying RD technology to catalytic chemical reactions: The use of RD technology is only possible if the temperature window of the vapor-liquid equilibrium is equivalent to the reaction temperature.
- The flexibility in the operating temperature of an RD column is not only restricted by the fact that two phases are required for the distillation process. Also, the thermal stability of the catalyst can limit the upper operating temperature.
- Because of the necessity of wet catalyst pellets, the chemical reaction must take place entirely in the liquid phase.
- As it is very expensive to change the catalyst in the structured packing of an RD column, only catalysts with a long lifetime are suitable for this process.