A zeolite is a naturally occurring microporous crystalline material based on silicon and aluminum that can b modified to be used in an ion exchange bed. There are other types of structures, some of which are organic synthetic materials, that are collectively and colloquially known as “resins” that are also capable of being used in ion exchange beds. Zeolites are a single type of ion exchange resin.
Hydrated cations within the zeolite pores are bound loosely to the zeolite framework, and can readily exchange with other cations when in aqueous media. Applications of this can be seen in water-softening devices and the use of zeolites in detergents and soaps. The largest volume use for zeolites is in detergent formulations where they have replaced phosphates as water-softening agents. They do this by exchanging the sodium in the zeolite for the calcium and magnesium present in the water. It is even possible to remove radioactive ions from contaminated water.
Types of zeolite
Zeolites are natural minerals that are mined in many parts of the world; most zeolites used commercially are produced synthetically. When developing applications for zeolites, it is important to remember that not all of these minerals are the same.
There are nearly 50 different types of zeolites (clinoptilolite, chabazite, phillipsite, mordenite, etc.) with varying physical and chemical properties. Crystal structure and chemical composition account for the primary differences. Particle density, cation selectivity, molecular pore size, and strength are only some of the properties that can differ depending on the zeolite in question. It is important to know the specific type of zeolite one is using in order to assure that it is appropriate for one’s needs.
There are numerous naturally occurring and synthetic zeolites, each with a unique structure. The pore sizes commercially available range from approximately 3 Å to approximately 8 Å. Some of the commercial materials are A, beta, mordenite, Y, ZSM-5.
The biggest differences between natural and synthetic zeolites are:
- Synthetics are manufactured from energy-consuming chemicals and naturals are processed from natural ore bodies.
- Synthetic zeolites have a silica-to-alumina ratio of 1 to 1 and clinoptilolite (clino) zeolites have a 5 to 1 ratio.
- Clino natural zeolites do not break down in a mildly acid environment, whereas synthetic zeolites do. The natural zeolite structure has more acid-resistant silica to hold its structure together. The clino natural zeolite is broadly accepted for use in the agricultural industry as a soil amendment and as a feed additive.
In 1948, Richard Barrer first produced a synthetic zeolite that did not have a natural counterpart. At approximately the same time, Milton made the first materials that had no natural counterpart such as zeolite A. New natural zeolites are still being discovered, and new synthetic zeolites are being invented in many laboratories around the world.
Principles of Zeolite Softening
The removal of hardness from water by a zeolite softening process is described by the following reaction:
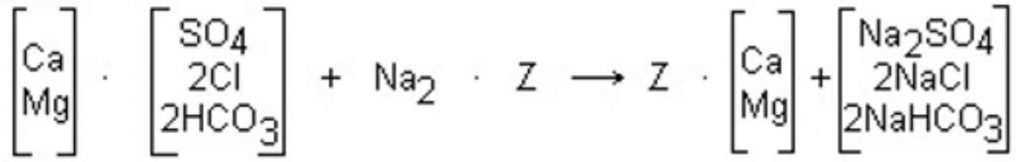
Water from a properly operated zeolite softener is nearly free from detectable hardness. However, some small amounts of hardness, known as leakage, are present in the treated water. The level of hardness leakage is dependent on the hardness and sodium level in the influent water and the amount of salt used for regeneration.
After the final rinse, the softener produces a low, nearly constant level of hardness until the ion exchange resin nears exhaustion. At exhaustion, the effluent hardness increases sharply, and regeneration is required.
As illustrated by the softening reactions, SAC resin readily accepts calcium and magnesium ions in exchange for sodium ions. When the exhausted resin is regenerated, a high concentration of sodium ions is applied to the resin to replace calcium and magnesium. The resin is treated with a 10% sodium chloride solution, and regeneration proceeds according to the following equation:
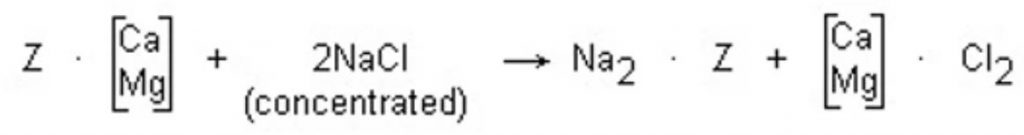
During regeneration, a large excess of regeneration (approximately 3 times the amount of calcium and magnesium in the resin) is used. The eluted hardness is removed from the softening unit in the waste brine and by rinsing.
After regeneration, small residual amounts of hardness remain in the resin. If the resin is allowed to sit in a stagnant vessel of water, some hardness will diffuse into the bulk water. Therefore, at the initiation of flow, the water effluent from a zeolite softener can contain hardness even if it has been regenerated recently. After a few minutes of flow, the hardness is rinsed from the softener, and the treated water is soft.
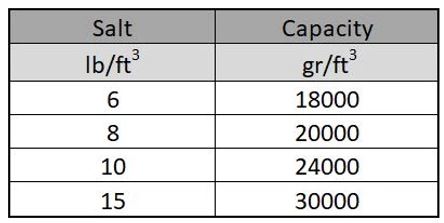
The duration of a service cycle depends on the rate of softener flow, the hardness level in the water, and the amount of salt used for regeneration. Table 8-1 shows the effect of regenerate level on the softening capacity of a regular strong cation resin. Note that the capacity of the resin increases as the regenerate dosage increases, but the increase is not proportional. The regeneration is less efficient at the higher regenerate levels. Therefore, softener operating costs increase as the regenerate level increases. As shown by the data in Table-1, a 150% increase in regenerate salt provides only a 67% increase in operating capacity.
Read also: Pump types, their specifications, and applications
Softener Operation
A sodium zeolite softener operates through two basic cycles: the service cycle, which produces soft water for use, and the regeneration cycle, which restores resin capacity at exhaustion.
In the service cycle, water enters the softener through the inlet distribution system and flows through the bed. The hardness ions diffuse into the resin and exchange with sodium ions, which return to the bulk water. Soft water is collected in the underdrain system and discharged. Service water flow to the softener should be as constant as possible to prevent sudden surges and frequent on-off operations.
Due to resin requirements and vessel designs, the softening operation is most efficient when a service flow rate between 6 and 12 GPM per square foot of resin surface area is maintained. Most equipment is designed to operate in this range, but some special designs utilize a deep resin bed to permit operation at 15-20 GPM/ft². Continuous operation above the manufacturer’s suggested limits can lead to bed compaction, channeling, premature hardness breakthrough, and hardness leakage.
Operating well below the manufacturer’s recommended flow rates can also negatively affect softener performance. At low flow rates, the water is not sufficiently distributed, and the optimum resin-water contact cannot take place.
When a softener is exhausted, the resin must be regenerated. Monitoring of the effluent hardness reveals resin exhaustion. When hardness increases, the unit is exhausted. Automatic monitors provide a more constant indication of the condition of the softener than periodic operator sampling and testing but require frequent maintenance to ensure accuracy. Many facilities regenerate softeners before exhaustion, based on a predetermined time period or the number of gallons processed.
Most softening systems consist of more than one softener. They are often operated so that one softener is in regeneration or standby while the other units are in service. This ensures an uninterrupted flow of soft water. Prior to placing a standby softener into service, the unit should be rinsed to remove any hardness that has entered the water during the standing time.
Hot zeolite softening
Zeolite softeners can be used to remove residual hardness in the effluent from a hot process lime or lime-soda softener. The hot process effluent flows through filters and then through a bed of strong acid cation resin in the sodium form. The equipment and operation of a hot zeolite softener are identical to that of an ambient temperature softener, except that the valves, piping, controllers, and instrumentation must be suitable for the high temperature (220-250 F).
Standard strong cation resin can be used at temperatures of up to 270 F, but for longer service life, a premium gel or macro reticular resin is recommended. When operating a zeolite system following a hot process softener, it is important to design the system to eliminate flow surges in the hot lime unit. Common designs include the use of backwash water storage tanks in the hot lime unit and extended slow rinses for the zeolite in lieu of a standard fast rinse.