There are five major methods to reduce BOD and COD
- Hydrogen peroxide as Oxidant
- ClO2 as Oxidant
- Microbiology Process
- Process Filtration and Adsorption with Activated Carbon
- COD Reducement using Precipitation Process (Coagulation)
1. Hydrogen peroxide as Oxidant
Hydrogen peroxide (H2O2) has been used to reduce the BOD and COD of industrial wastewaters for many years. While the cost of removing BOD and COD through chemical oxidation with hydrogen peroxide is typically greater than that through physical or biological means, there are nonetheless specific situations that justify the use of hydrogen peroxide.
Read also: Permit to Work System
- Pre-treatment of high strength / low flow wastewaters – where biotreatment may not be practical prior to discharge to a Publicly Owned Treatment Works (POTW);
- Enhanced separation of entrained organics by flotation and settling processes; and
- Supply of supplemental Dissolved Oxygen (DO) when biological treatment systems experience temporary overloads or equipment failure.
As indicated by these examples, H2O2 can be used as a stand-alone treatment or as an enhancement to existing physical or biological treatment processes, depending on the situation.
1.1. Direct Chemical Oxidation Using Hydrogen Peroxide
Hydrogen peroxide can be used alone or with catalysts – such as iron (Fe2+ or Fe3+), UV light, ozone (O3), and alkali – to oxidize BOD/COD contributing compounds in wastewaters. The type of oxidation needed depends on the type of BOD/COD present. This relationship is present in the figure below.
If a large fraction of the BOD and COD is contributed by inorganic reduced sulfur compounds such as sulfides, sulfides, or thiosulfate, then hydrogen peroxide alone is typically effective. Depending on the wastewater pH, the oxidation of these compounds by H2O2 yields sulfate or colloidal sulfur, neither of which contributes to BOD and COD. If the primary contributors to BOD and COD are dissolved organics, then a more reactive oxidation system is needed. Moderate activation of hydrogen peroxide can be achieved by:
- alkali (generating the per hydroxyl ion, OOH- – the active agent in peroxide bleaching systems);
- certain transition metals (e.g., tungstate, vanadate, molybdate) which form reactive peroxometal complexes in-situ;
- certain mineral acids (e.g., sulfuric) which form reactive peroxyacid derivatives such as peroxymonosulfuric acid (Caro’s Acid) ex-situ.
For the more recalcitrant organics such as chlorinated solvents, extremely reactive free radical systems (termed Advanced Oxidation Processes) are needed. A generalized reaction using Fenton’s Reagent for reducing BOD and COD can be expressed as follows:
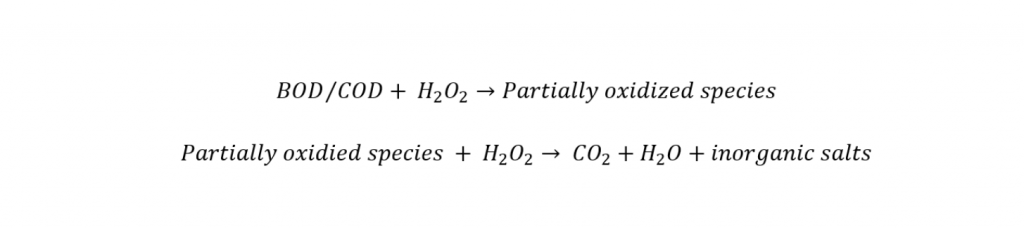
The extent of oxidation typically depends on the amount of hydrogen peroxide used. The theoretical hydrogen peroxide requirement is about 2.1 lbs (as 100%) per lb-BOD and COD oxidized. In many cases, however, complete digestion of the organic compounds to carbon dioxide and water is not needed. Partial oxidation to intermediate compounds minimizes chemical consumption and often results in substantial reductions in BOD and COD and toxicity.
1.2. Enhanced physical separation of BOD and COD with Hydrogen Peroxide
Enhanced physical separation of BOD and COD with hydrogen peroxide may occur is two ways.
In the first case, partial oxidation of organic contaminants results in more polar (charged) substances which are more agreeable to adsorption onto coagulants and flocculants. As illustrated in the example below, this allows BOD and COD removal efficiencies with less than stoichiometric hydrogen peroxide doses.
In the second case, enhanced physical separation (flotation) of fats, oils, and greases (FOG) is provided by H2O2. This occurs by the natural decomposition of hydrogen peroxide to oxygen and water, i.e., hydrogen peroxide will supersaturate the wastewater with oxygen, which results in the formation of evenly dispersed microbubbles that scavenge FOG constituents as they rise to the surface of the water. In some cases, this can increase BOD removal through dissolved air flotation cells from e.g., 50% to 90-95%. Typical doses are 25-100 mg/L H2O2, the cost for which can often be offset against savings in coagulant use – a polyelectrolyte polymer is generally still needed.
2. ClO2 as Oxidant
Chlorine dioxide (ClO2) which was first used in drinking water treatment is an oxidant increasingly of interest for industrial and wastewater treatment (Eckenfelder and Bowers, 1994). It has become a disinfectant alternative to chlorine and ozone because it offers the prospect of disinfection without the production of trihalomethanes and bromates so it is gaining acceptance as a water treatment promise. As an oxidant, ClO2 is generally considered for reuse strategies, because it is more effective than chlorine in the inactivation of most pathogens. Besides, its biocidal properties are not influenced by pH and it is a more powerful oxidant than chlorine.
2.1. Generation of CLO2
There are several methods available for ClO2 generation. For this study, the direct acid system has been used because it was well studied for most small treatment systems (White, 1992). This method utilizes the reaction of a strong acid with sodium chlorite (NaClO2). The following reaction which is most acceptable for H2SO4 is:
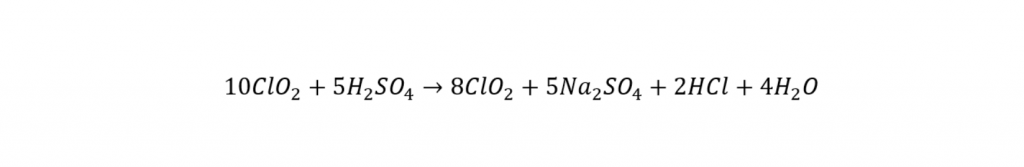
As was already mentioned, ClO2 gas produced in the direct acid system was absorbed in enough distilled water. The ClO2 concentration thus, prepared can be determined by the iodometric method as outlined in Standard Methods (APHA, 1995). However, yield determination can be done only when a laboratory apparatus for the determination of chlorite and chlorate ions are available.
Assessment of advanced treatment of secondary effluent by ClO2 shows the considerable effect of this gas in reducing the remained pollutants of these effluents. Moreover, ClO2 has been shown to be an effective disinfectant for bacterial indicators. Safe production of ClO2 is possible by the direct acid system and required amounts for effluent polishing would be reduced by modifying the performance of treatment plants to have a typic effluent at all times and even further by upgrading them to produce effluents with a new standard of 10/10 for example by employing a filtration step prior to ClO2 injection.
ClO2 treatment seems to be an acceptable treatment technology to ensure good effluent quality for reuse applications such as agricultural and landscape irrigation and even for all the other urban non-potable applications when it is employed for filtered effluents. One final but important point for ClO2 treatment concerns the required time.
3. Microbiology Process
COD reduction process by the method of using bacteria or microorganisms, devoted to COD derived from organic matter with a high content of biodegradable. This process is done in two main ways,
- Aeration
- Anaerobic
In the aeration process, COD is reduced by making the bacteria can break down organic compounds in water. These bacteria are called heterotrophic bacteria that break down organic compounds due to the use of oxygen. This process is usually used in wastewater with a COD of less than 3000 mg / L.
In the process of anaerobic bacteria work in a room with minimal oxygen content. This process is also called fermentation, in which bacteria break down autotroph working with organic compounds from wastewater with three stages of one of them is by taking oxygen from organic compounds. The anaerobic process is suitable for wastewater with BOD levels over 2000 mg / L.
Before deciding whether to take this method, it is important for you to understand what types of wastewater that you face. Because the microbiological process is only suitable for wastewater with organic contents. You can determine this by looking at the comparison between COD and BOD.
4. Process Filtration and Adsorption with Activated Carbon
This process is used after the primary treatment of wastewater to reduce the COD and BOD of water. Usually used as an activated carbon filter. Activated carbon will absorb organic substances, ozone, or chlorine substances remaining on the processing results. So safe effluent to be discharged into the environment. Filtration using activated carbon is also commonly used in water treatment processes to remove odors and reduce the chemicals in the water.
5. COD Reducement using Precipitation Process (Coagulation)
Most of the COD is sourced from the TSS or no dissolved solids commonly called sludge. The most important way to get rid of the sludge is to use coagulants and flocculants. The principle is to bind the sludge to one another so that a larger clump of sludge and then be deposited in a sedimentation tank.
Some chemicals commonly used as a coagulant, among others; PAC, FeCl3 (Ferric Chloride), and Alum.
This deposition process will greatly affect the value of COD. Especially in wastewater with a high enough amount of TSS. COD reduction process in order to become more perfect, it is advisable to give more concern to the process of mixing and sedimentation. Due to the proper mixing process, the precipitation reaction that occurs will be less than perfect. Some processes similar to this is the process of using MBR, SBR, or SMBR Reduce COD with Oxidation.
Some chemicals can help you to reduce the COD of wastewater. Chlorine, Hydrogen peroxide, and Ozone will oxidize chemicals in the water so that the automatic COD value will go down. But of course, you should limit dose usage of oxidation, because oxidation possesses sufficient affection, especially for living organisms. COD reduction technique is suitable for waste has COD values derived from non-biodegradable waste such as Phenol, surfactants, etc.