The world is dependent on hydrocarbon fuels for a long time. These hydrocarbons are extracted from conventional fossil fuels. To fulfill the global energy demand, researchers are consistently working to exact the fossil fuel from unconventional reservoirs. Some recent development in the field able us to recover the fuel from the tight and shale formations. However, there is still a potential unconventional source of natural gas that lies beneath the sea which is termed Methane Hydrates. These reserves of hydrates are believed to be a larger hydrocarbon resource than all the world’s Coal, Oil, and Natural gas combined. If these deposits can be extracted economically, they can become the energy savior for the world.
What is Methane Hydrate?
It is a crystalline solid that contains a methane molecule surrounded by an interlocking cage of water molecules. Methane hydrate is an “ice” that naturally occurs in the subsurface deposits where the temperature and pressure conditions are favorable for its formation.
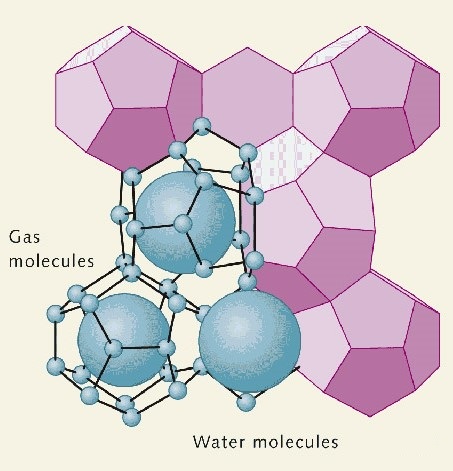
If the ice is removed from its existing environment, it becomes highly unstable. For this reason, methane hydrate deposits are very difficult to study. They cannot be drilled for a study like other subsurface materials. When they are brought to the surface, the pressure is reduced and the temperature rises which causes the ice to melt and the methane to escape from its cage.
Methane hydrates are also known as methane clathrate, hydromethane, methane ice, fire ice, natural gas hydrate, and gas hydrate.
Deposits of Methane Hydrate?
There are four Earth environments that have the temperature and pressure conditions suitable for the formation and stability of these hydrates.
- Sediment and sedimentary rock units below Arctic permafrost
- Sedimentary deposits along continental margins
- Under deep-water sediments of inland lakes and seas
- Under the Antarctic ice.
Methane hydrates accumulations are not very deep except in the Antarctic deposits. It can be found within a few hundred meters of the sediment surface in most situations.
In these environments, methane hydrate occurs in the sediment as layers and intergranular cement. The deposits are so dense and persistent that they create an impermeable layer that traps natural gas moving upwards from below.
Methane Hydrate Hazards
Methane hydrates are very sensitive sediments. They can rapidly dissociate with a decrease in pressure or an increase in temperature. This dissociation produces free methane and water. The conversion of solid sediment into liquids and gases will cause a loss of support and shear strength. These can cause powerful submarine slumping and landslides that can damage production equipment and pipelines.
Moreover, methane is a powerful greenhouse gas (GHG). Warmer Arctic temperatures could result in the gradual melting of these gas hydrates. The warming oceans could cause the gradual melting of the gas hydrates near the sediment-water interface. Although many reports have presented this as a potential catastrophe. The USGS research has determined that gas hydrates are currently contributing to total atmospheric methane and that a catastrophic melting of unstable hydrate deposits is unlikely to send large amounts of methane into the atmosphere.

Energy Potential
Although the accumulations of the methane hydrates are located in difficult environments and present numerous technical challenges. A variety of technologies need to be developed to produce them either by using pressure reduction or ion exchange that takes advantage of their different chemical and physical properties. Countries like the United States, Canada, and Japan all have research programs working to discover viable technologies for producing these hydrates. Methane hydrate will likely play a vital role in our future energy mix.
Read also: Steam Methane Reforming Process