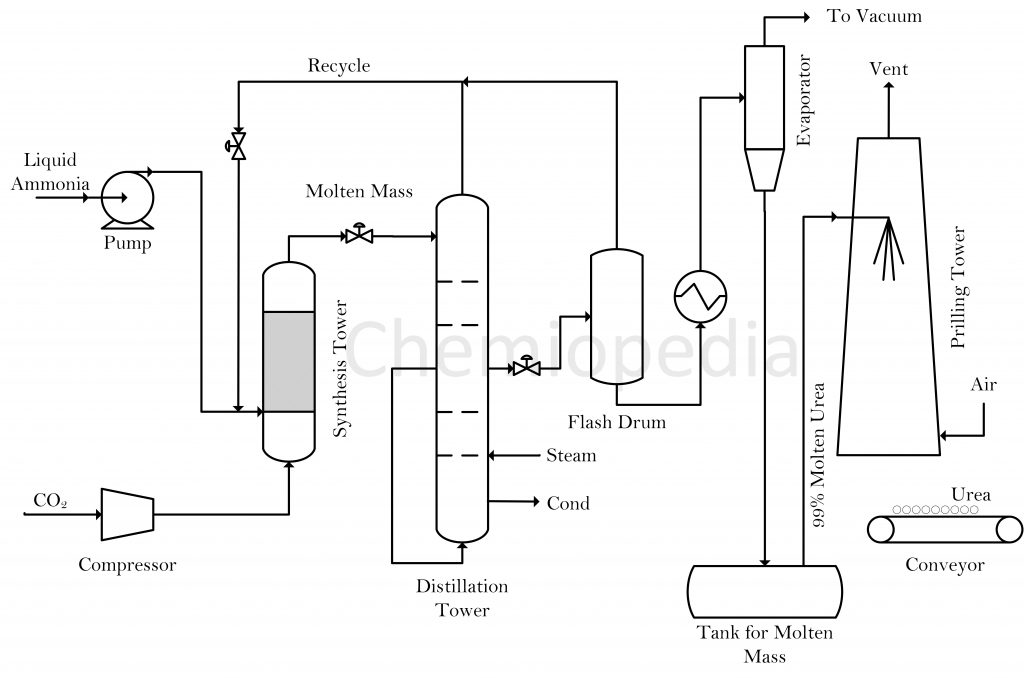
Urea manufacturing involves two reactants, ammonia and carbon dioxide. Carbon dioxide is produced as a byproduct of the ammonia plant, hence utilized in the downstream urea manufacturing unit. The reactants first go into a synthesis reactor in which liquid ammonia and compressed carbon dioxide reacted to produce urea. Ammonia and carbon dioxide react to produce Ammonium Carbamate:
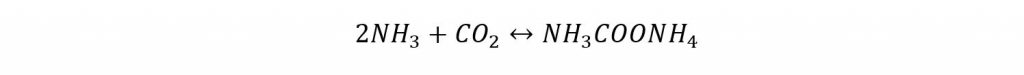
The pressure and temperature are maintained at 14 Mpa and 170-190°C for the first reaction to occur. The reaction is highly exothermic in nature. Most of the heat released is utilized in form of process steam wherever it is needed in to process.
Read also: Nitrogen rejection process
The unreacted ammonia, carbon dioxide, and product from the reactor go to the decomposer where Ammonium Carbamate converts into Urea by the following reaction.
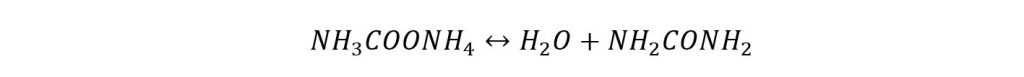
It is an endothermic reaction, hence, requires certain energy to proceed. Biuret can also be formed as a byproduct if the temperature rise is excessive.
The unreacted ammonia and carbon dioxide separated in decomposer and flash vessel. These gases are recycled back to the synthesis reactor. The overall conversion of the process can be as high as 99% but per pass conversion of the reactor is limited to 50% due to certain design challenges.
The molten mass from the reactor and decomposer contains unreacted ammonia, carbon dioxide, ammonium carbamate, and water. These impurities are removed using a distillation tower and evaporator. The essential condition is to keep the temperature high and pressure low during stages of separation. At these conditions, the ammonium carbamate decomposes back to ammonia and carbon dioxide.
These gases are then removed from the flash and recycled back to the reactor. The evaporator then concentrates the mixture by evaporating the excess water in the mixture. The molten urea then passes through nozzles inside the prilling tower. Compressed air is passed in the tower so that its flow is counter-current with respect to that of molten urea. The urea gets solidified in the prilling tower and air helps in shaping it in the form of prills or granules. The Urea is then stored and ready to be sold.