Carbon dioxide, hydrogen sulfide, and other contaminants are often found in natural gas streams. CO2 when combined with water creates carbonic acid which is corrosive. CO2 also reduces the BTU value of gas and in concentrations of more than 3 %, the gas is unmarketable.
On the other hand, H2S is an extremely toxic gas that is also tremendously corrosive to equipment. Amine sweetening processes remove these contaminants so that the gas is marketable and suitable for transportation.
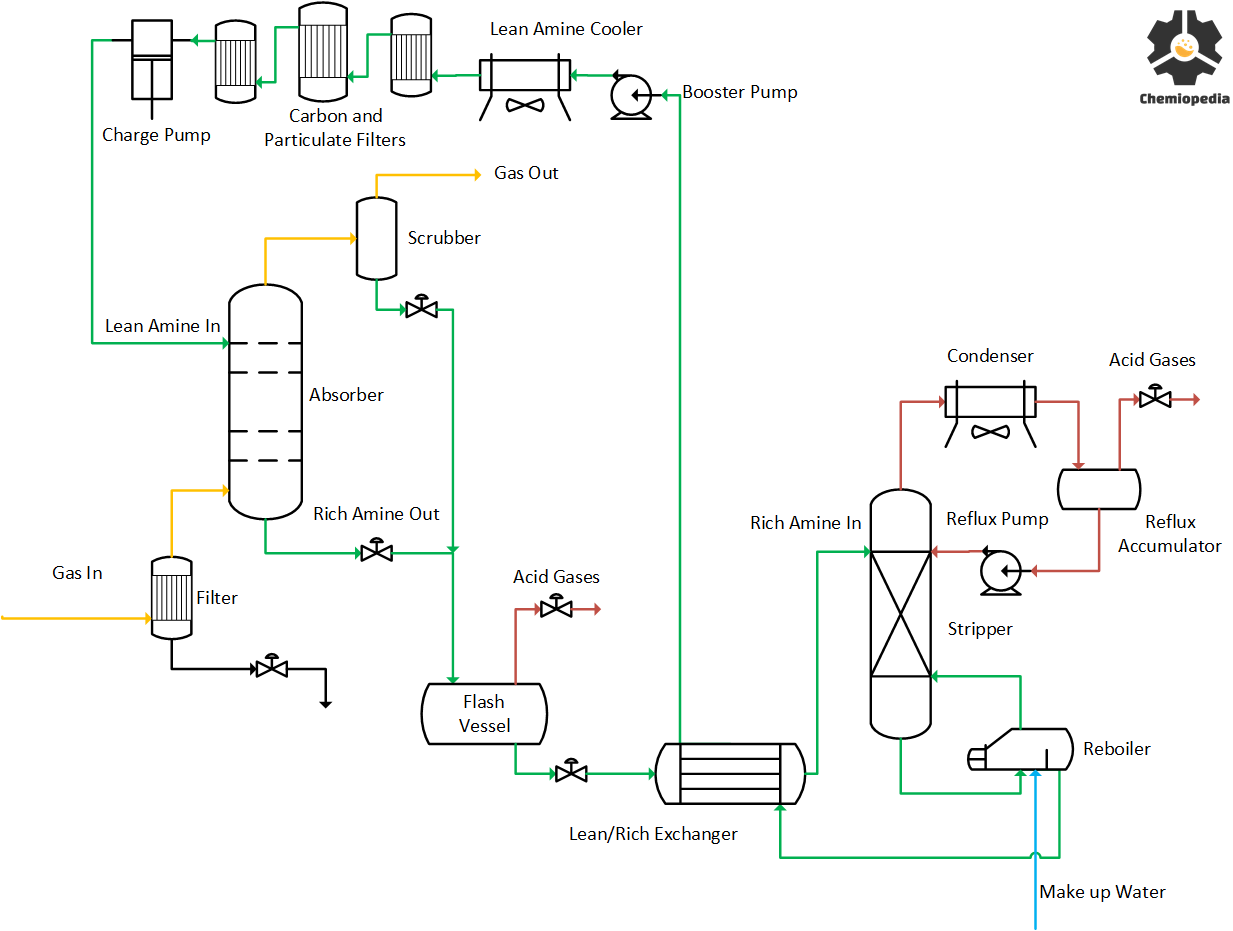
Read More: Amine Sweetening Process
The categorization trend of acid gas removal processes keeps changing with the invention of new technologies. Keeping in view all of them so far developed acid gas treating processes, they have been divided into the following categories:
1. Physical Processes of Acid Gas Removal
In addition to the chemical solvents, there are also physical solvents available for extracting the acid gases from natural gas. Physical solvents do not react chemically with acid gases but have a high physical absorptive capacity. The amount of acid gas absorbed is proportional to the partial pressure of the solute, and no upper limit, owing to saturation, is evident, as is the case with chemical solvents. Hence, they are mainly suited for sour gases with high acid gas content at high contacting pressures. The physical absorption solvents have the advantage of regeneration by flashing upon reduction of pressure and, therefore, do not require much heat in the stripping column. This makes physical solvents useful as bulk-removal processes, followed by final cleanup using a chemical solvent because physical solvents have difficulty in achieving the H2S limit specified for sales gas. Unfortunately, they also tend to absorb heavier hydrocarbons, which is a disadvantage if the acid gas is fed to a Claus plant for sulfur recovery.
1.1. Selexol Process
The Selexol process was developed by Allied Chemical Corp. The solvent is dimethyl ether of polyethylene glycol and is usually used in its pure form. It is more selective towards H2S than CO2, and, therefore, some CO2 remains in the gas stream, depending on the mole loading of the solvent. Several stages of flashing are provided for in the regeneration step, to allow the absorbed hydrocarbons to evolve from the solution. The flashed gases from the initial flash stages are compressed and returned to the inlet of the absorber. Selexol is noncorrosive and also removes water vapor from the gas stream.
1.2. Fluor Process
The Fluor Solvent Process is patented by Fluor Corporation. This process uses propylene carbonate for the removal of CO2 and H2S from natural and synthesis gas streams. Propylene carbonate, C4H6O3, is a polar solvent that has a high affinity for CO2. Propylene carbonate is anhydrous, non-corrosive, non-toxic, biodegradable, and is readily available from several solvent manufacturers.
1.3. Rectisol Process
The Rectisol Process is licensed by LINDE AG. The Rectisol process uses methanol (CH3OH) for removal of acid gases by physical absorption at relatively low temperatures. The process is used for removal of H2S, CO2, HCN, NH3, gum-forming hydrocarbons, higher hydrocarbons, and other impurities from natural gas. It is also widely used in conjunction with low-temperature plants for hydrogen purification and separation of light hydrocarbons.
1.4. Purisol Process
The Purisol Process is licensed by Lurgi AG. The Purisol process uses N-Methyl-2-Pyrrolidone C5H9NO (NMP or M-Pyrol) as a solvent. Since NMP has a high solubility for H2S and CO2, chemical reactions are not required. Typically, the process is used for
- Reduction of high CO2 concentrations to a low level
- Bulk removal of acidic components to a moderate level with a simplified flash regenerator system
- Selective H2S removal.
2. Chemical Solvent Process
2.1. Flexorb Process
The Flexorb Process is licensed by Exxon Research & Engineering. Flexsorb is a family of solvents that uses a new class of amines called hindered amines. These solvents are used for selectively removing H2S, CO2, and bulk removal of H2S and CO2 respectively. The solvent offers advantages such as no corrosion and no foaming compared to regular amine solvents.
2.2. Benfield Process
The Benfield Process is licensed by UOP. Benfield process is a thermally regenerated cyclical solvent process that uses an activated, inhibited hot potassium carbonate solution to remove CO2, H2S, and other acid gas components. The process can be flexibly being designed for pressures from 100-2000 psia for acid gas content of a few percent up to 50%. The Benfield process is based on chemical reactions of potassium carbonate and acid gases.
2.3. Catacarb Process
The Catacarb Process is licensed by Eickmeyer & Associates. This process uses catalytically aided hot potassium carbonate (K2CO3) solution to remove acid gases by chemical absorption. Catacarb solution contains catalysts to promote the absorption-desorption reactions, and corrosion inhibitor is also added to the solution to mitigate the corrosion problems.
The absorption rate of potassium carbonate solution increases with the temperature. The catalyst additive promotes the chemical reaction rates in both the absorption and regeneration steps. This enables the Catacarb solution to be regenerated to a much lower level than is found in the ordinary hot-potassium carbonate process.
Typical acid gas compositions treated in the hydrogen and ammonia plants are 15-30%. This range is 5-50% acid gas for natural gas sweetening applications. The concentration of Catacarb solution is normally 20-30 wt%.
2.4. UOP Amine Guard Process
The Amine Guard Process is licensed by UOP. This technology combines the high-performance, formulated, family of solvents with the reliable Amine Guard system process technology. There thermally regenerated and/or flash regenerated solvent process removes CO2, H2S, and other sulfur components. Hydrocarbon losses are minimal due to their low solubility in the solvent. The process incapable of achieving very low product gas specifications, to 1 ppm of H2S and 50 ppm of CO2.
2.5. Amine Sweetening Process
The amine sweetening process is the process used to remove acid gases like CO2 and H2S from natural gas. The process selected depends upon the different factors. Some of these factors are given below:
- H2S and mercaptan concentration in the sour gas
- Sales gas H2S and total sulfur limits
- Sales gas CO2 limit
- Gas flow rate
3. Physical/Chemical Solvent
The physical/chemical solvent processes use mixtures of chemical and physical solvents to enhance the treating efficiency. Two such processes are available; Shell Sulfinol Process and Amisol Process. The physical/chemical solvent processes include:
3.1. Shell Sulfinol Process
Shell Sulfinol Process is licensed by Shell. This process was originally developed for treating gases very rich in H2S & CO2. It uses a mixture of an organic physical solvent called Sulfolane C4H8O2S (tetrahydrothiophene dioxide) mixed with an alkanol amine (CH3)2HC-NH-CH(CH3)2 (di-isopropanolamine, DIPA), which is a chemical absorbent, and water. Shell Sulfinol process can effectively remove H2S, CO2, COS, CS2, mercaptans, and organic sulfides and disulfides from feed gas streams.
3.2. Amisol Process
The Amisol Process is licensed by the Lurgi AG. The Amisol process uses a combination of chemical and physical solvents. The chemical solvent is an amine; the most common combinations being DIPA (di-isopropylamine) (CH3)2HC-NH-CH(CH3)2 or DEA (diethanolamine) HN(CH2CH2OH)2. The physical solvent used is methanol.
The process bears several advantages over simple amines or simple methanol processes. It can be designed for the bulk removal of acid gases. It can remove H2S, COS, CO2.
4. Membrane Processes
Membranes have become an established technology for carbon dioxide removal since their first use in this application in 1981. Initial acceptance was slow and limited to smaller streams, mostly because of the economic risks involved in treating larger streams, but also because many process design parameters were largely unknown.
Membranes have been widely used in two main CO2 removal applications:
- Natural gas sweetening
- Enhanced Oil Recovery (EOR), where CO2 is removed from an associated natural gas stream and reinjected into the oil well to enhance oil recovery.
Membranes are thin semi-permeable barriers that selectively separate some compounds from others. This definition is necessarily broad because of the large variety of membrane materials separating an equally vast number of compounds in all phases.
Currently, the only commercially viable membranes used for CO2 removal are polymer-based, for example, cellulose acetate, polyimides, polyamides, polysulfone, polycarbonates, and polyetherimide. The most widely used and tested material is cellulose acetate. Polyimide has some potential in certain CO2 removal applications, but it has not received sufficient testing to be used in large applications. The Membrane processes include:
4.1. Separax Membrane
Separax membrane is provided by UOP for the treatment of Acid Gases. It employs cellulose acetate polymer and It is suitable for the treatment of gas because of higher hydrocarbon recovery.
4.2. MTR Membranes
These membranes are provided by Membrane Technology and Research. This process has a low hydrocarbon recovery, which consequently leads to a higher feed flow rate. Also, the MTR membrane process does not meet the sales gas water and H2S specification.
4.3. Generon IGS, Inc.
Generon IGS membranes are being able to operate at a lower feed pressure and being able to provide better performance at the lower feed pressure because of the superior separation properties, i.e. selectivity of CO2/CH4.
5. Molecular Sieve Process
The molecular sieve process uses an adsorbent to remove the acid gas based on the selective adsorption of the acid gas molecules. Hydrocarbons do not adsorb and just pass through the adsorbent bed. Most molecular sieve adsorbents are composed of aluminosilicate crystalline polymer. They efficiently remove polar or polarizable contaminants such as water, methanol, H2S, CO2, COS, mercaptans, sulfides, ammonia, and mercury down to trace concentrations.
Molecular sieve adsorbents are regenerative through Pressure Swing (PS). The commercially available molecular sieve technology is Molecular Gate Technology. Molecular Gate uses proprietary adsorbents for selective removal of CO2 and H2S.
Molecular Gate Technology is based on the principle of molecular sizes. The molecular size of H2S, CO2, and N2 is smaller and that of methane is larger. The size of the pores is so selected that can adsorb H2S and CO2. Methane molecules, being larger in size, would not be able to adsorb and just pass through.
5.1. Pressure Swing Adsorption
Pressure swing adsorption (PSA) is a technology used to separate some gas species from a mixture of gases under pressure according to the species’ molecular characteristics and affinity for an adsorbent material. It operates at near-ambient temperatures and differs significantly from cryogenic distillation techniques of gas separation. Specific adsorptive materials are used as a trap, preferentially adsorbing the target gas species at high pressure. The process then swings to low pressure to desorb the adsorbed material.
5.1.1. Process
Pressure swing adsorption processes utilize the fact that under high pressure, gases tend to be attracted to solid surfaces, or “adsorbed”. The higher the pressure, the more gas is adsorbed. When the pressure is reduced, the gas is released or desorbed. PSA processes can be used to separate gases in a mixture because different gases tend to be attracted to different solid surfaces more or less strongly. If a gas mixture such as air is passed under pressure through a vessel containing an adsorbent bed of zeolite that attracts nitrogen more strongly than oxygen, part or all of the nitrogen will stay in the bed, and the gas exiting the vessel will be richer in oxygen than the mixture entering. When the bed reaches the end of its capacity to adsorb nitrogen, it can be regenerated by reducing the pressure, thus releasing the adsorbed nitrogen. It is then ready for another cycle of producing oxygen-enriched air.
This is the process used in medical oxygen concentrators used by emphysema patients and others requiring oxygen-enriched air for breathing.
Using two adsorbent vessels allows near-continuous production of the target gas. It also permits so-called pressure equalization, where the gas leaving the vessel being depressurized is used to partially pressurize the second vessel. This results in significant energy savings and is a common industrial practice.
6. Centrifugal Processes
This process is one of the latest innovations in the gas treating industry. Shell has developed a process for the removal of acid gases from natural gas streams that uses centrifugal forces. This technique uses liquefaction of CO2 at elevated pressure and low temperature and is followed by centrifugal separation. This is purely a unit operation and involves no chemical consumption or reaction.
There are two steps in this process. The first step is the cooling step in which the gas is cooled by expansion through turbine expander to a temperature such that CO2 forms micron-sized liquid droplets. The second step is the separation of the mist by using a rotational particle separator. The process uses a turbine expander to expand the gas to cool it. In order to liquefy CO2, a combination of pressure and temperature greater than the triple point of CO2 (i.e. 74 psia and -70°F) is needed.
After the gas is liquefied to micron-sized droplets, the liquid particles are removed from the gas by using a rotating particle separator. The separator core is a cylindrical body housing a large number of axially oriented channels of 1-2 mm diameter. The swirling high velocity of the gas induces the rotation of the assembly which is mounted on bearings. Thus, the rotating pipe separator does not need an external motor drive.
The energy recovered by the turbine can be used for the compression of the treated gas. One of the other advantages of this process is that CO2 and H2S are produced at high pressure enabling re-injection into the same reservoir from which it originated.
4 Comments
Selexol Process – Chemiopedia · June 5, 2021 at 3:06 pm
[…] See: Natural Gas Sweetening Processes […]
Rectisol Process – Chemiopedia · June 5, 2021 at 3:07 pm
[…] See: Natural Gas Sweetening Processes […]
Purisol Process – Chemiopedia · June 5, 2021 at 3:17 pm
[…] See: Natural Gas Sweetening Processes […]
Fluor Process - Chemiopedia · June 14, 2022 at 4:25 pm
[…] See: Natural Gas Sweetening Processes […]
Comments are closed.